Our Science
What are T Regulatory Cells?
- Regulatory T cells (“Tregs”) are T cells that act as a peacekeeper. They regulate or suppress “bad players” in the immune system.
- Tregs could be viewed as the “control switch” of the body’s immune response to self and foreign particles, or antigens.
- Tregs are key for “self tolerance”: in other words, to prevent excessive immune responses and autoimmunity.
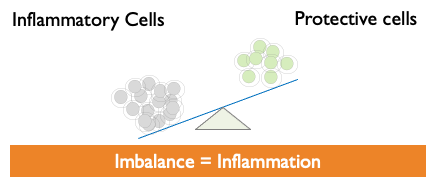
- Defective or decreased Treg cells can lead to immune imbalance, which can then result in uncontrolled inflammation and various autoimmune diseases.
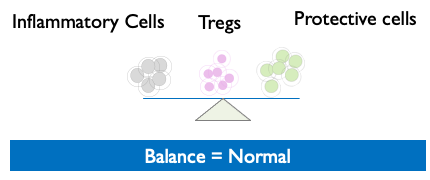
- Treg cell therapy can allow the immune system to regain control and return to “normal,” thereby resolving inflammatory issues.
Tregs restore Equilibrium
What makes Cellenkos Tregs Unique?
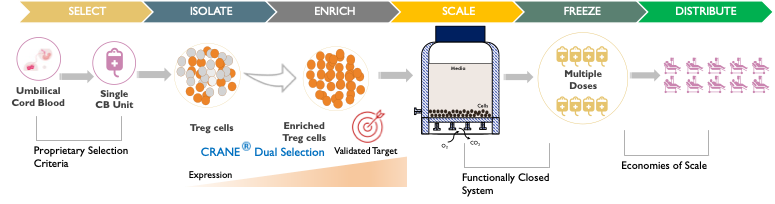
Cellenkos Technology extracts the bonafide suppressor Treg cells derived from the healthy umbilical cord blood, and using its proprietary CRANE® technology, is able to generate Treg cells that can expand inside the body.
Cellenkos’ Treg cells utilizes the body’s internal GPS to seek out inflammatory hotspots and then quickly leave the circulation to preferentially accumulate in the target inflamed tissue. Upon arrival to the tissue, Cellenkos’ Treg cells leverage their cellular intelligence to dial up or dial down the suppressor function in order to adapt to the local microenvironment. This works to restore the immune equilibrium back to normal. Such dual property as well as self-regulation differentiates Cellenkos Treg cells from all others. Cellenkos’ transformational platform is being developed to generate therapeutics to treat a wide spectrum of diseases driven by inflammatory processes.
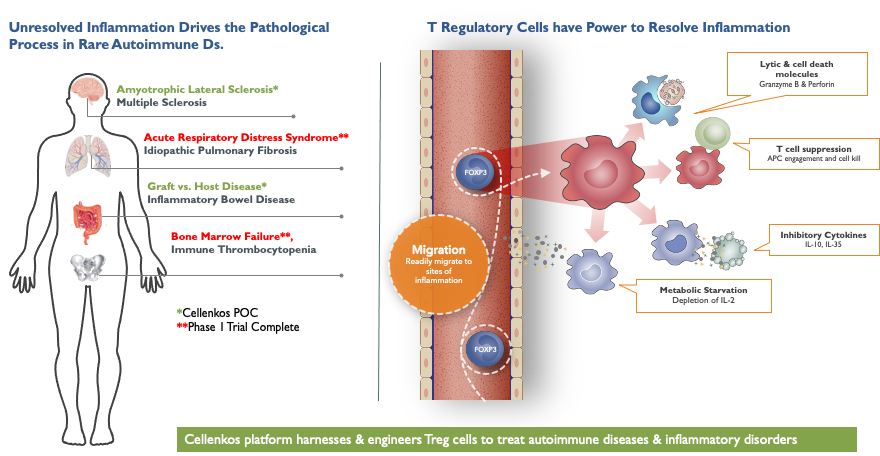
The are main advantages of Cellenkos’ Treg cells that make them an ideal therapeutic substance include:
No Plasticity
Treg cells extracted from an adult patient carry the problem of duplicity where the cell may carry the risk of flipping into a harmful T effector cell (Th17) based on the tissue microenvironment. Cellenkos Treg cells are hardwired to remain suppressive even under the toughest circumstances due to the lack of the expression of ROR𝛾t, which is responsible for Th17 cell production.
Low Immunogenicity
Cellenkos Treg cells are derived from the healthy allogeneic umbilical cord blood and are therefore very young, naive cells that carry no memory. This allows the Tregs to easily bypass the scrutiny of the recipient’s immune system and therefore escape immune rejection. Such lack of markers allows for the Cellenkos Treg cells to be infused without the need for matching with the patient. This qualifies Cellenkos’ Tregs as an ideal source for adoptive cell therapy.
Surplus Availability
Cellenkos uses umbilical cord blood already stored in the qualified public cord blood banks as a source for its Treg cells and has access to more than 1 million cord blood units currently in storage. Such surplus supply inventory can continuously support the development of various Treg cell therapeutic approaches.
Drug Depot Manufacturing
Cellenkos Technology generates a large-scale expansion of Treg cells from a single starting cord blood unit. These cell products are then cryopreserved in ready-to-use cryobags and can be stored at subzero temperatures for years. Such frozen Treg cells can be thawed by the patient’s bedside and infused at the time of need.
CRANE® Platform
Cord Blood T-Regulatory Cells; Activated And Enriched.
Cellenkos’ proprietary CRANE® technology allows for cell enrichment and differentiation such that each product is tailored to a specific disease.
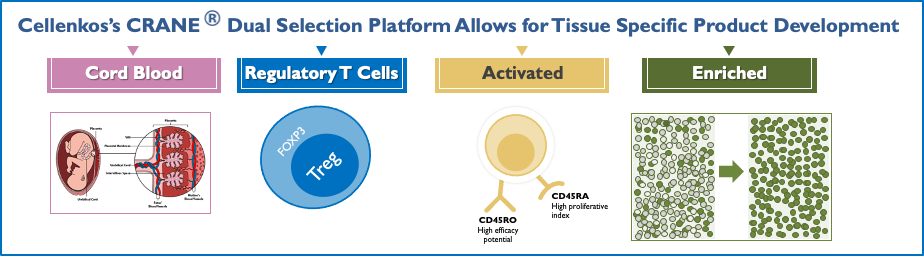
Such product characterization allows for its own regulatory filings and independent commercialization pathway.
cGMP Manufacturing
Cellenkos possesses in-house cGMP manufacturing infrastructure and expertise such that the technology transfer, process development and scale up occurs at the stand alone, company owned, Cellenkos Manufacturing Facility (CMF) located in Houston, TX.

Control on supply chain, process development and distribution allows for seamless product development, storage and distribution.

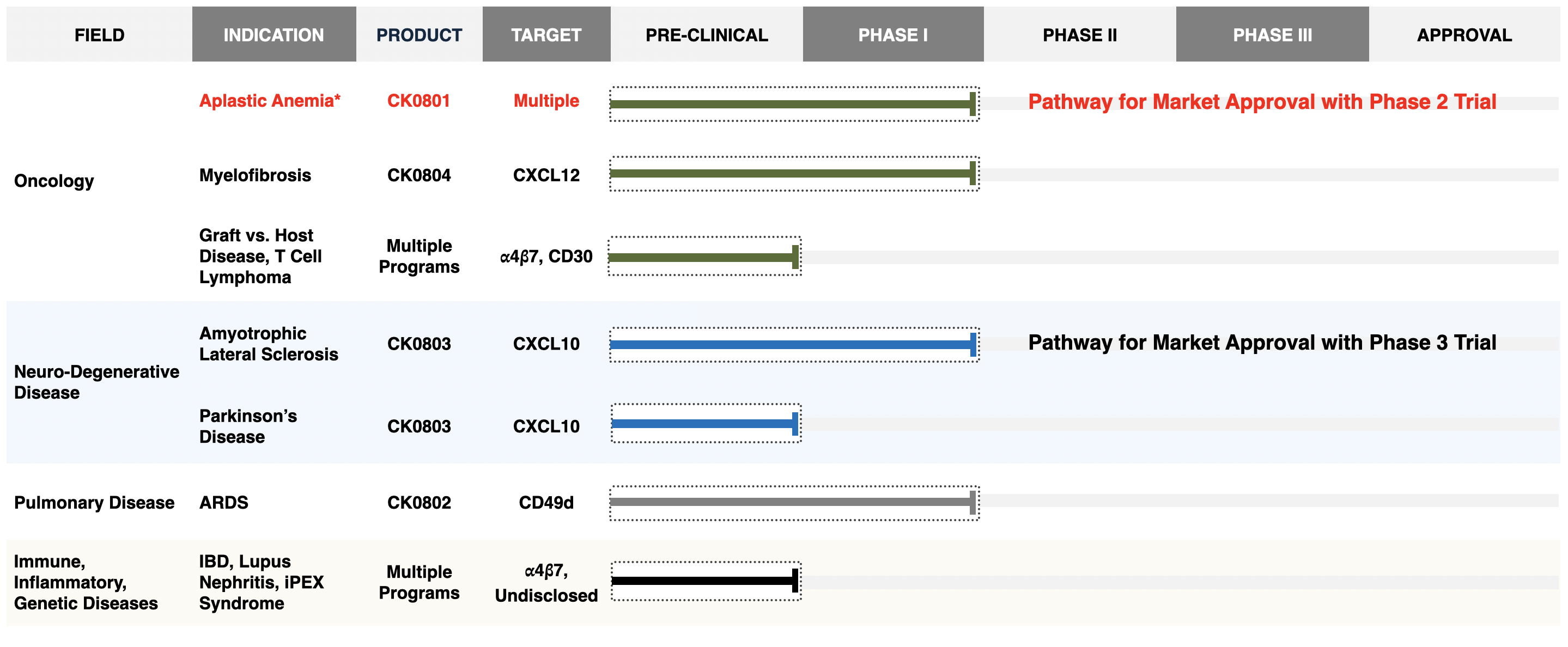
Pipeline
Cellenkos has a robust clinical pipeline whose near-term clinical success will drive growth. The platform technology allows for deep R&D that holds promise for the future. Cellenkos has established its identity as a Stand-Alone Enterprise and created meaningful relationships with leading academic centers for diverse therapeutic sleeves.
Clinical Trials
CK0801: Bone Marrow failure
Phase I Trial to Evaluate the Safety and Feasibility of CK0801 in Treatment of Bone Marrow Failure Syndrome
Link: https://clinicaltrials.gov/ct2/show/NCT03773393?term=cellenkos&draw=2&rank=2
CK0802: COVID-19 ARDS
Phase 1 Double-Blinded, Randomized, Placebo Controlled Safety and Early Efficacy Trial of Cryopreserved Cord Blood Derived T-Regulatory Cell Infusions (CK0802) In The Treatment Of COVID-19 Induced Acute Respiratory Distress Syndrome (ARDS)
Link: https://clinicaltrials.gov/ct2/show/NCT04468971?term=cellenkos&draw=2&rank=1
CK0804: Myelofibrosis
Phase Ib, Open-label Study of Add on Therapy With CK0804 in Participants With Myelofibrosis, With Suboptimal Response to Ruxolitinib
Link: https://clinicaltrials.gov/ct2/show/NCT05423691?term=cellenkos&draw=2&rank=1
CK0803: Amyotrophic Lateral Sclerosis
Phase 1 Safety Run-in Study of 6 patients followed by Phase 1b Randomized, Double Blind, Placebo Control Trial of CK0803, neurotropic, allogeneic, umbilical cord blood derived T regulatory (Treg) cells in additional 60 patients with Amyotrophic Lateral Sclerosis.